
All other known metals (iron, copper, silver, gold, zinc, mercury, tin, lead and bismuth) had no recorded discoverers. Cobalt became the first metal to be discovered since the pre-historical period. He showed that compounds of cobalt metal were the source of the blue color in glass, which previously had been attributed to the bismuth found with cobalt. Swedish chemist Georg Brandt (1694–1768) is credited with discovering cobalt circa 1735, showing it to be a previously unknown element, distinct from bismuth and other traditional metals. Because the primary ores of cobalt always contain arsenic, smelting the ore oxidized the arsenic into the highly toxic and volatile arsenic oxide, adding to the notoriety of the ore. Discovery: Cobalt compounds have been used since the Bronze Age to impart a blue color to glass. The first attempts to smelt those ores for copper or silver failed, yielding simply powder (cobalt(II) oxide) instead. Cobalt is atomic number 27 with element symbol Co on the periodic table.
The word cobalt is derived from the German kobalt, from kobold meaning "goblin", a superstitious term used for the ore of cobalt by miners.

Too much or too little cobalt in the body can cause health issues.Cobalt-60 is used to create gamma rays which are used to treat cancer and to sterilize medical supplies.Cobalt was the first metal to be discovered since prehistoric times and the first metal with a recorded discoverer.The most common oxidation states are +2 and +3. It is also a component of the vitamin B 12.Ĭobalt gets its name from the German word "kobalt" which means "goblin." Miners gave cobalt ore this name as they were superstitious about mining the ore.Ĭobalt only has one stable isotope that is found in nature: cobalt-59.Ĭobalt exists with oxidations states ranging from -3 to +4. The body uses it to create certain enzymes. He isolated the element and proved that it was the source of the color in blue glass which previously was thought to be from bismuth.Ĭobalt compounds were used throughout ancient history by civilizations such as Ancient China and Rome to make blue glass and ceramics.Ĭobalt is also important for animal life. Other applications for cobalt include batteries, industrial catalysts, electroplating, and powerful magnets.Ĭobalt was discovered by Swedish chemist George Brandt in 1735. Most of the cobalt that is mined is used in superalloys which are very resistant to corrosion and are stable at high temperatures.Ĭobalt is also used as a blue coloring agent in paints, inks, glass, ceramics, and even cosmetics. The majority of cobalt is mined in Africa and is a byproduct of the mining of other metals including nickel, copper, silver, lead, and iron. Cobalt ores include erythrite, cobaltite, skutterudite, and glaucodot.
#COBALT ATOMIC FREE#
It forms many compounds with other elements such as cobalt(II) oxide, cobalt(II) fluoride, and cobalt sulfide.Ĭobalt is not found as a free element, but is found in minerals in the Earth's crust. It reacts slowly with oxygen from the air. It can be easily magnetized and maintains its magnetism at high temperatures.Ĭobalt is only somewhat reactive. It is one of the few elements that is naturally magnetic.
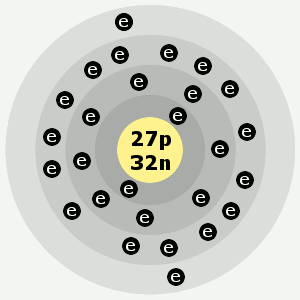
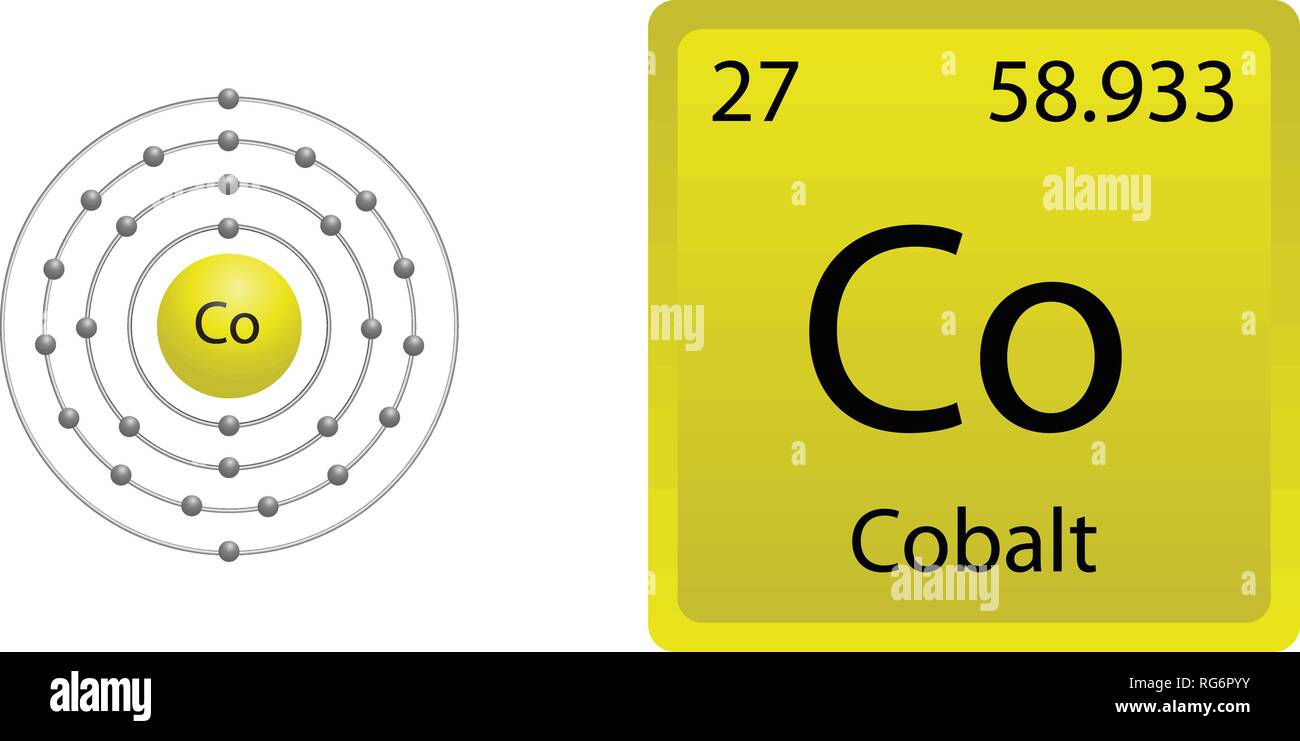
Under standard conditions cobalt is a hard, brittle metal with a bluish-white color. Cobalt atoms have 27 electrons and 27 protons with 32 neutrons in the most abundant isotope. Cobalt is the first element in the ninth column of the periodic table.


 0 kommentar(er)
0 kommentar(er)
